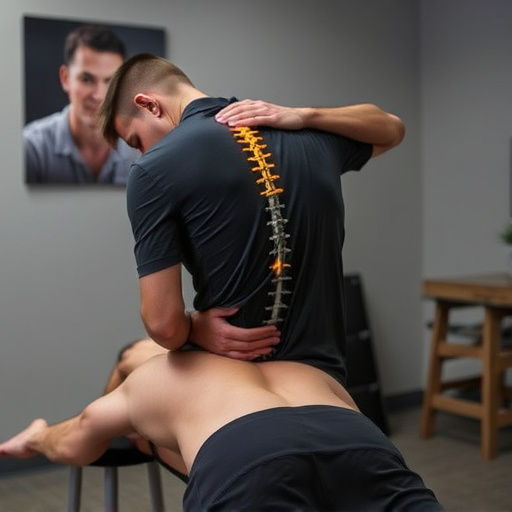
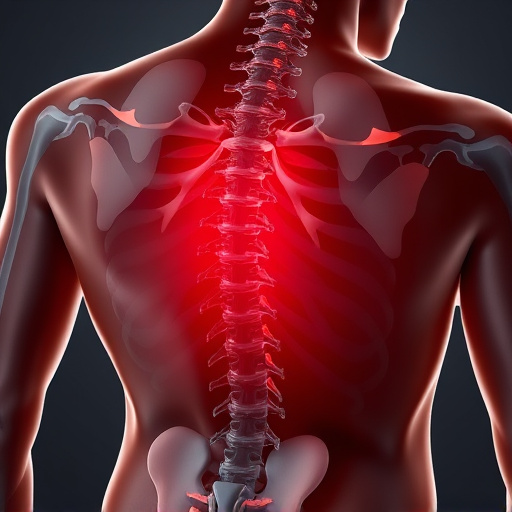
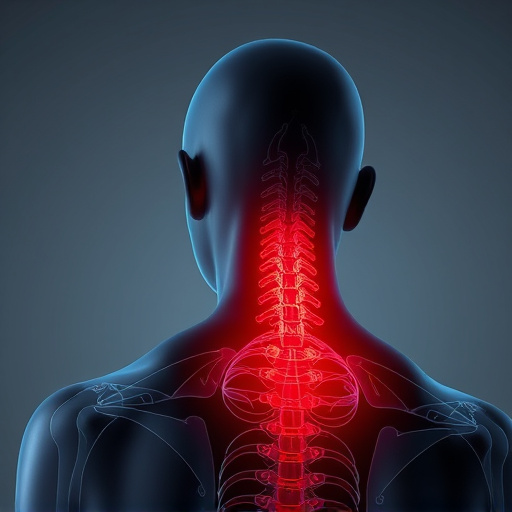
Shockwave therapy for pain is a non-invasive, FDA-approved treatment using low-energy sound waves to heal musculoskeletal injuries, including tendinopathy, muscle strains, and arthritis. Effective for athletes and rehabilitation, it accelerates healing, improves mobility, and reduces inflammation with minimal side effects, offering an appealing alternative to surgery or medication.
“Discover how FDA-approved shockwave therapy emerges as a game-changer in pain management. This non-invasive treatment is transforming the way we address chronic conditions, offering relief where traditional methods fall short.
In this comprehensive guide, we explore the science behind shockwave therapy, its rigorous FDA approval process, and its remarkable benefits for various painful ailments. From muscle injuries to joint pain, learn how this innovative approach can revolutionize your quality of life.”
- Understanding Shockwave Therapy for Painful Conditions
- FDA Approval Process and Its Significance in Pain Management
- Common Uses and Benefits of Non-Invasive Shockwave Treatment
Understanding Shockwave Therapy for Painful Conditions

Shockwave therapy for pain is a non-invasive treatment that uses low-energy sound waves to promote healing and alleviate discomfort associated with various conditions. This advanced technology has gained recognition within the medical community for its potential in treating chronic musculoskeletal pain, including issues like tendinopathy, muscle strains, and joint arthritis. By delivering targeted acoustic energy, shockwave therapy can stimulate blood flow, enhance tissue repair, and reduce inflammation, offering a promising alternative to conventional treatments.
In the context of spinal adjustments and sports injury recovery, shockwave therapy has shown effectiveness in accelerating rehabilitation processes. It is particularly beneficial for athletes and individuals facing challenges in injury rehabilitation, as it can aid in restoring mobility and strength. Moreover, its non-surgical nature makes it an appealing option for those seeking alternative solutions to conventional pain management approaches, including physical therapy or medication.
FDA Approval Process and Its Significance in Pain Management
The U.S. Food and Drug Administration (FDA) plays a pivotal role in ensuring the safety and effectiveness of medical devices, including shockwave therapy systems for pain relief. Before a device can be marketed, it must undergo rigorous testing and evaluation to meet the FDA’s stringent standards. This process involves preclinical studies to assess the device’s mechanics and potential risks, followed by clinical trials involving human subjects to demonstrate its efficacy and safety in treating specific conditions related to shockwave therapy for pain.
FDA approval is significant in the field of pain management as it signifies that a device has undergone extensive scrutiny. This process ensures that healthcare professionals and patients can have confidence in the technology’s performance, particularly when considering mobility improvement and sports injury treatment. The FDA’s oversight helps maintain high-quality standards, fostering advancements in medical devices while prioritizing patient safety.
Common Uses and Benefits of Non-Invasive Shockwave Treatment
Shockwave therapy for pain has gained significant attention due to its non-invasive nature and proven benefits. This innovative treatment utilizes low-intensity acoustic waves to stimulate tissue repair and promote healing, making it a popular choice for managing various conditions. One of the most common uses is in addressing musculoskeletal injuries, where shockwave therapy can accelerate the natural recovery process and reduce inflammation.
Beyond its effectiveness in treating sports-related injuries, this therapy has shown promise in providing headache relief and chronic pain management. By targeting specific areas, it can offer significant improvements to patients suffering from persistent discomfort. The non-invasive approach makes it an attractive alternative to more invasive procedures, allowing individuals to experience relief with minimal side effects.
Shockwave therapy for pain has emerged as a non-invasive treatment option backed by FDA approval, offering significant benefits for various painful conditions. By understanding the science behind this innovative approach and its regulatory significance, patients and healthcare providers can leverage the power of shockwave therapy to alleviate symptoms and improve overall quality of life. Its common uses range from musculoskeletal disorders to vascular issues, making it a versatile tool in modern pain management.